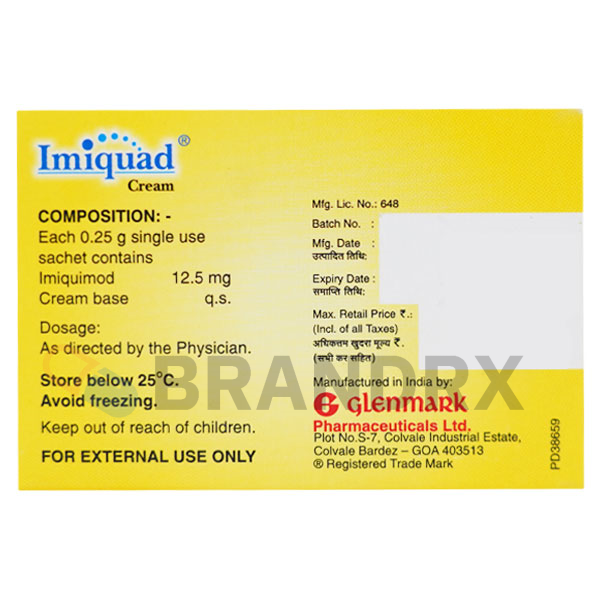
Additional information
| Active substance | Imiquimod |
|---|---|
| Hepatotoxicity | Low risk (minimal systemic absorption) |
| Lab Test | None specific for monitoring Imiquimod |
| Strength | 5% |
| Also known as | Imiquimod cream |
| Blood pressure | No effect |
| Trade name | Aldara, Zyclara |
| Storage conditions | Store at room temperature away from moisture and heat |
| Chemical name | 1-(2-methylpropyl)-1H-imidazo[4,5-c]quinolin-4-amine |
| Formula | C14H16N4 |
| Substance class | Immune response modifier |
| Main action | Induces immune response through Toll-like receptor 7 (TLR7) agonism |
| Half-life | Not applicable for topical administration (systemic absorption minimal) |
| Dosage (medical) | Apply topically to the affected area as per the prescription, usually 2 to 3 times per week |
| Dosage (sports) | Not applicable |
| Effects | Enhances local immune response to treat skin conditions |
| Side effects | Local skin irritation, redness, itching, flakiness, and in rare cases, systemic reactions |
| Use in sports | None |
| Manufacturer | Glenmark Pharmaceuticals Ltd. |
| Packing | 3 sachets/pack |




Reviews
There are no reviews yet.